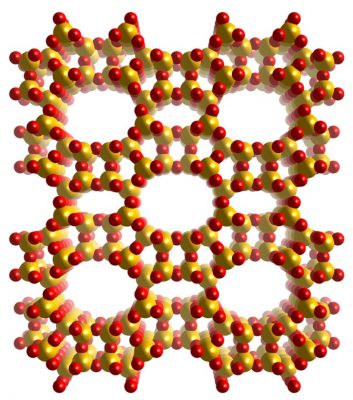
Z
eolites are porous aluminosilicates used in a great number of catalytic and purification processes. Although only few elements are involved in the formation of zeolites, the flexibility of the T-O-T angles (T=Si, Al) allows for a great number of different polymorphs to be formed.
As of today, the International Zeolite Association (IZA) recognizes more than 240 zeolite frameworks.
These materials present an extreme variability in terms of both topology and aluminum content, and post-synthetic treatments can introduce further structural features.
One example of this is the exchange of the cations present in the structures: these species are needed to compensate for the extra negative charge introduced by Al3+ ions compared to Si4+ ones, and can be alkali metals, transition metals, H+ ions and many more. In their protonic form, zeolites possess Brønsted acidic properties and can therefore be used as solid acid catalysts.
Upon exchange, the counterion can act as a Lewis acid and/or a redox agent, opening possibilities for different applications in catalysis, purification and storage.
Cu-zeolites: a way to stabilize reactive intermediates
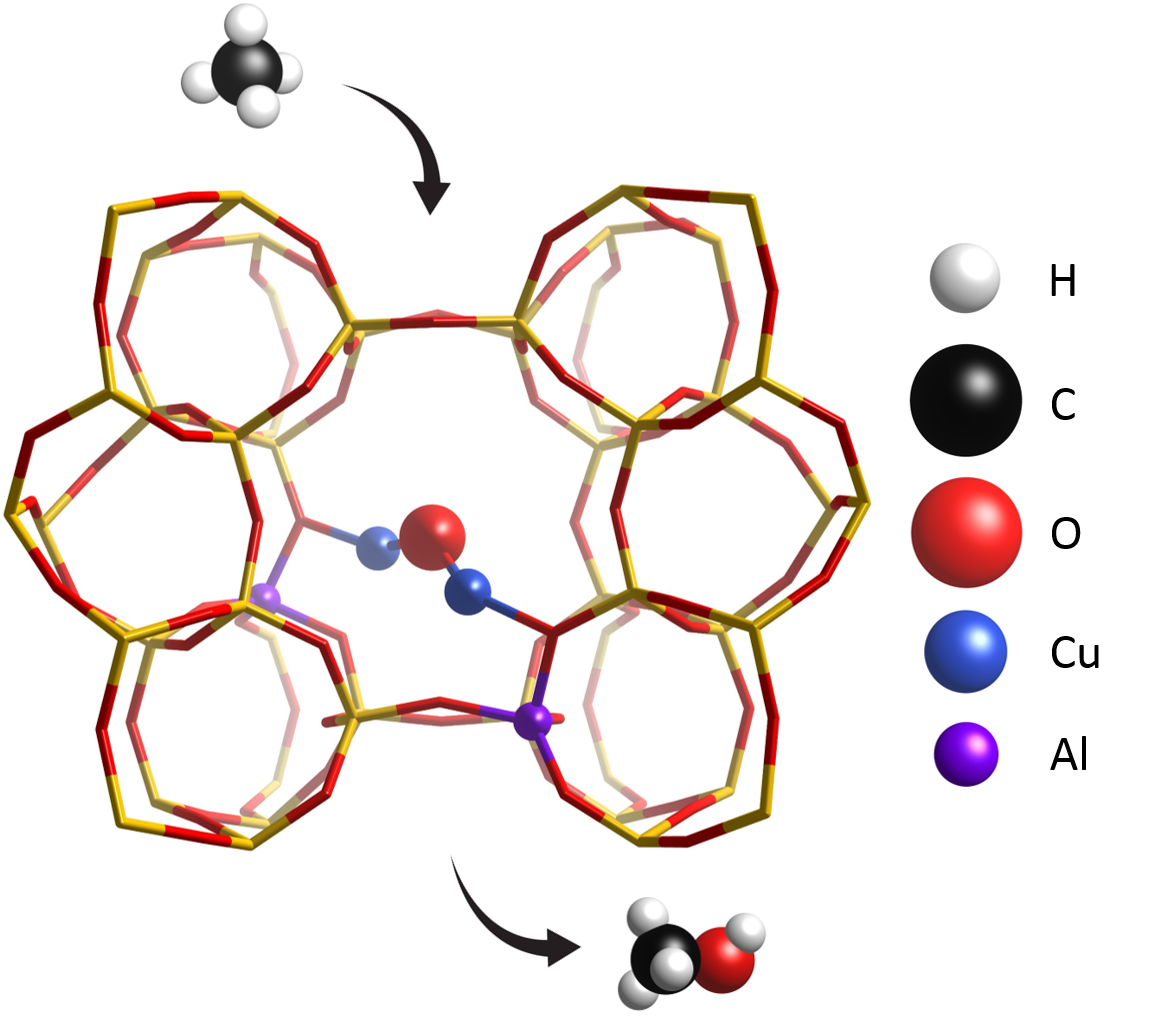
I
n 2005, Schoonheydt’s group found that in the Cu-exchanged ZSM-5 zeolite methane can be selectively converted to methanol with a specific treatment in O2. CH4 is very susceptible to overoxidation, since its partially oxidized analogues tend to be more reactive than itself towards CO2; the active species that form in these materials can, however, stabilize some intermediates (e.g, methoxy groups) via chemisorption, significantly boosting selectivity. The involvement of Cu-oxo species in this mechanism has been confirmed with many techniques, but as of today no general consensus has been reached on the exact identity of the species; among the candidates, multi-meric oxygen-bridged clusters have been detected and well characterized for many systems by many groups. Apart from composition and topology, activation protocols also play an important role in this process, as differences in temperature and pressure can lead to different results.
Understanding the secret to the outstanding selectivity of Cu-zeolites towards methane oxidation can lead to a general understanding of the key features a material should possess to carry out this reaction in an industrially viable way, thus paving the way towards rational engineering of a new generation of catalysts.
For more information check these articles:
Groothaert, M. H.; Smeets, P. J.; Sels, B. F.; Jacobs, P. A.; Schoonheydt, R. A. Selective Oxidation of Methane by the Bis(μ-Oxo)Dicopper Core Stabilized on ZSM-5 and Mordenite Zeolites. J. Am. Chem. Soc. 2005, 127, 1394–1395.
Pappas, D. K.; Borfecchia, E.; Dyballa, M.; Pankin, I. A.; Lomachenko, K. A.; Martini, A.; Signorile, M.; Teketel, S.; Arstad, B.; Berlier, G.; et al. Methane to Methanol: Structure-Activity Relationships for Cu-CHA. J. Am. Chem. Soc. 2017, 139, 14961–14975.
Martini, A.; Borfecchia, E.; Lomachenko, K.A.; Pankin, I.A.; Negri, C.; Berlier, G.; Beato, P.; Falsig, H.; Bordiga, S.; Lamberti, C.; Composition-driven Cu-speciation and reducibility in Cu-CHA zeolite catalysts: a multivariate XAS/FTIR approach to complexity. Chem. Sci., 2017, 8, 6836-6851.
Snyder, B.E.R; Bols, M.L.; Schoonheydt, R.A.; Sels, B.F.; Solomon, E.I.; Iron and Copper Active Sites in Zeolites and Their Correlation to Metalloenzymes. Chem. Rev. 2018, 118, 5, 2718–2768.
Pappas, D. K.; Martini, A.; Dyballa, M.; Kvande, K.; Teketel, S.; Lomachenko, K. A.; Baran, R.; Glatzel, P.; Arstad, B.; Berlier, G.; Lamberti, C.; Bordiga, S.; Olsbye, U.; Svelle, S.; Beato, P.; Borfecchia, E.; The Nuclearity of the Active Site for Methane to Methanol Conversion in Cu-Mordenite: A Quantitative Assessment . J. Am. Chem. Soc. 2018, 140, 15270–15278.
Borfecchia, E.; Beato, P.; Svelle, S.; Olsbye, U.; Lamberti, C.; Bordiga, S.; Cu-CHA – a model system for applied selective redox catalysis. Chem. Soc. Rev., 2018,47, 8097-8133.
Sushkevich, V. L.; Van Bokhoven, J. A. Methane-to-Methanol: Activity Descriptors in Copper-Exchanged Zeolites for the Rational Design of Materials. ACS Catal. 2019, 9, 6293–6304.
Ravi, M.; Sushkevich, V.L.; Knorpp, A.J.; Newton, M.A.; Palagin, D.; Pinar, A.B.; Ranocchiari, M.; van Bokhoven, J.A.; Misconceptions and challenges in methane-to-methanol over transition-metal-exchanged zeolites. Nat.. Catal. 2019, 2, 485–494.
Newton, M. A.; Knorpp, A. J.; Sushkevich, V. L.; Palagin, D.; van Bokhoven, J. A. Active Sites and Mechanisms in the Direct Conversion of Methane to Methanol Using Cu in Zeolitic Hosts: A Critical Examination. Chem. Soc. Rev. 2020, 49, 1449-1486.
Tao, L.; Lee, I.; Sanchez-Sanchez, M.; Cu oxo nanoclusters for direct oxidation of methane to methanol: formation, structure and catalytic performance. Catal. Sci. Technol., 2020, 10, 7124-7141.