ACS Omega – Kelsi R. Hall, Maja Mollatt, Zarah Forsberg, Ole Golten, Lorenz Schwaiger, Roland Ludwig, Ivan Ayuso-Fernandez, Vincent G. H. Eijsink and Morten Sørlie
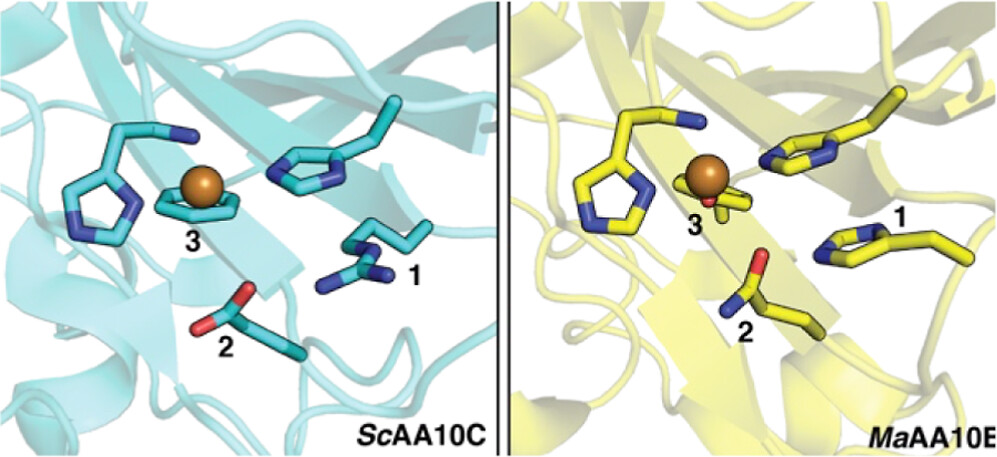
Lytic polysaccharide monooxygenases (LPMOs) catalyze the oxidative cleavage of glycosidic bonds in recalcitrant polysaccharides, such as cellulose and chitin, using a single copper cofactor bound in a conserved histidine brace with a more variable second coordination sphere. Cellulose-active LPMOs in the fungal AA9 family and in a subset of bacterial AA10 enzymes contain a His-Gln-Tyr second sphere motif, whereas other cellulose-active AA10s have an Arg–Glu–Phe motif. To shine a light on the impact of this variation, we generated single, double, and triple mutations changing the His216–Gln219–Tyr221 motif in cellulose- and chitin-oxidizing MaAA10B toward Arg–Glu–Phe. These mutations generally reduced enzyme performance due to rapid inactivation under turnover conditions, showing that catalytic fine-tuning of the histidine brace is complex and that the roles of these second sphere residues are strongly interconnected. Studies of copper reactivity showed remarkable effects, such as an increase in oxidase activity following the Q219E mutation and a strong dependence of this effect on the presence of Tyr at position 221. In reductant-driven reactions, differences in oxidase activity, which lead to different levels of in situ generated H2O2, correlated with differences in polysaccharide-degrading ability. The single Q219E mutant displayed a marked increase in activity on chitin in both reductant-driven reactions and reactions fueled by exogenously added H2O2. Thus, it seems that the evolution of substrate specificity in LPMOs involves both the extended substrate-binding surface and the second coordination sphere.
Click here for the complete article!