Catalysis Today – Barbara Centrella, Mouhammad Abu Rasheed, Matteo Bonomo, Alessandro Damin, Francesco D’Amico, Unni Olsbye, Claudia Barolo and Silvia Bordiga.
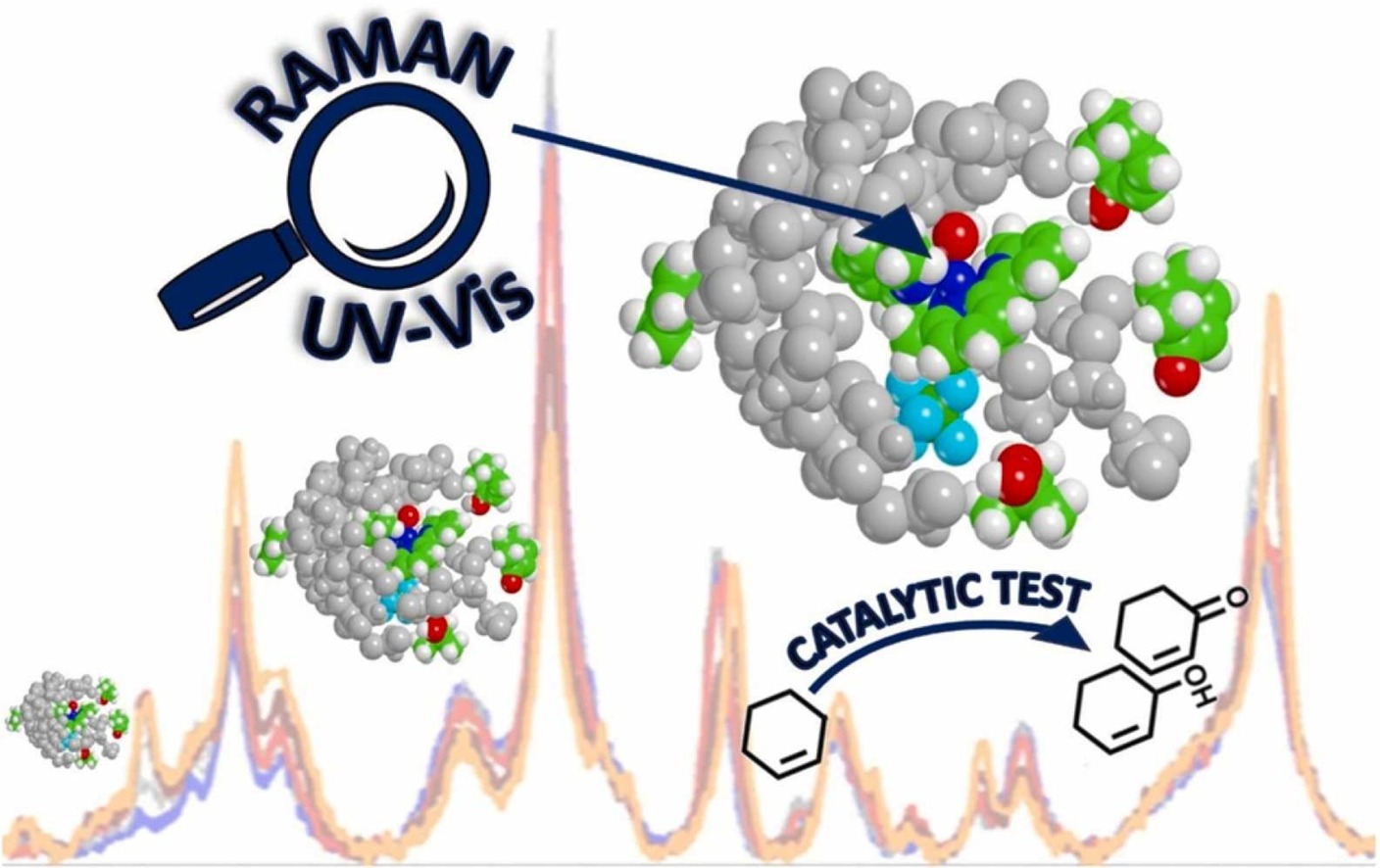
In Cu(6,6’-dimethyl-2,2’-bipyridine)2 (hereafter coded as CuBPA) the presence of methyl moieties allows a stabilization of Cu(I) form, enabling an easier switching between Cu(I) and Cu(II) that is of particular interest in catalysis. In this contribution, resonant Raman spectroscopy (with simultaneous UV-Vis acquisition) supported by DFT calculations is employed as a lens to precisely monitor the metal organic complex throughout its redox cycle and to identify its active species. As a model reaction, the CuBPA-mediated oxygenation of cyclohexene (as the reductant/substrate) by means of tert-Butyl hydroperoxide (as the oxidant) is selected. In this context, 266 nm laser turns out to be quintessential for the characterization of the oxidized form (Ox-CuBPA) and to follow the system evolution throughout the redox cycle, although it fails in giving an unambiguous identification and quantification of reaction products. Aiming at this, catalytic tests are performed under conditions that replicate the spectroscopic experiments. 2-cyclohexene-1-ol and 2-cyclohexene-1-one are identified as the dominant products, proving a promising catalytic activity of CuBPA in partial oxidation reactions.
Click here for the complete article!