Journal of the American Chemical Society – Kelsi R. Hall, Chris Joseph, Ivan Ayuso-Fernandez, Ashish Tamhankar, Lukas Rieder, Rannei Skaali, Ole Golten, Frank Neese, Asmund K. Røhr, Sergio A, V, Jannuzzi, Serena De Beer, Vincent G. H. Eijsink and Morten Sørlie
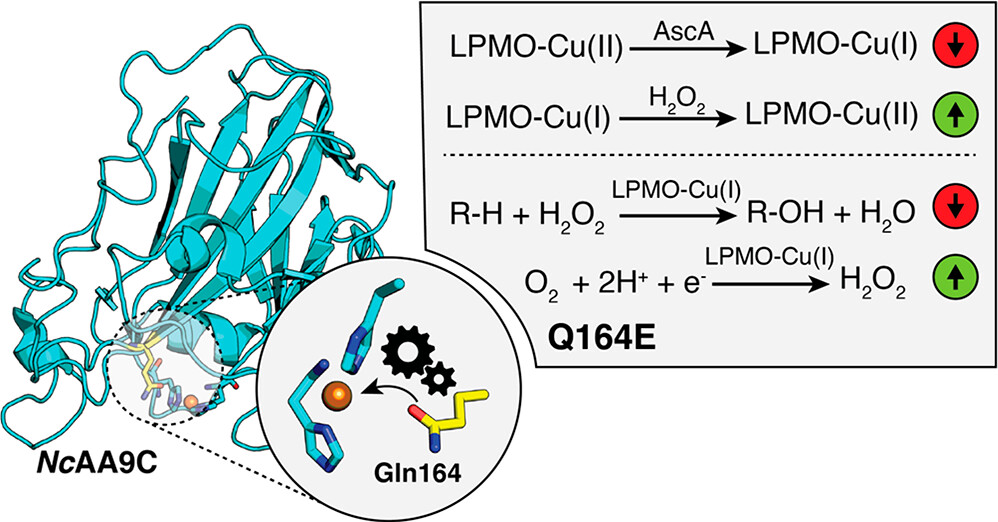
Lytic polysaccharide monooxygenases (LPMOs) are powerful monocopper enzymes that can activate strong C–H bonds through a mechanism that remains largely unknown. Herein, we investigated the role of a conserved glutamine/glutamate in the second coordination sphere. Mutation of the Gln in NcAA9C to Glu, Asp, or Asn showed that the nature and distance of the headgroup to the copper fine-tune LPMO functionality and copper reactivity. The presence of Glu or Asp close to the copper lowered the reduction potential and decreased the ratio between the reduction and reoxidation rates by up to 500-fold. All mutants showed increased enzyme inactivation, likely due to changes in the confinement of radical intermediates, and displayed changes in a protective hole-hopping pathway. Electron paramagnetic resonance (EPR) and X-ray absorption spectroscopic (XAS) studies gave virtually identical results for all NcAA9C variants, showing that the mutations do not directly perturb the Cu(II) ligand field. DFT calculations indicated that the higher experimental reoxidation rate observed for the Glu mutant could be reconciled if this residue is protonated. Further, for the glutamic acid form, we identified a Cu(III)-hydroxide species formed in a single step on the H2O2 splitting path. This is in contrast to the Cu(II)-hydroxide and hydroxyl intermediates, which are predicted for the WT and the unprotonated glutamate variant. These results show that this second sphere residue is a crucial determinant of the catalytic functioning of the copper-binding histidine brace and provide insights that may help in understanding LPMOs and LPMO-inspired synthetic catalysts.
Click here for the complete article!