The Journal of Physical Chemistry Letters – Gabriele Deplano, Isabelle Gerz, Derya Demirbas, Barbara Centrella, Matteo Bonomo, Serena DeBeer, Silvia Bordiga, Matteo Signorile, and Sergio A. V. Jannuzzi
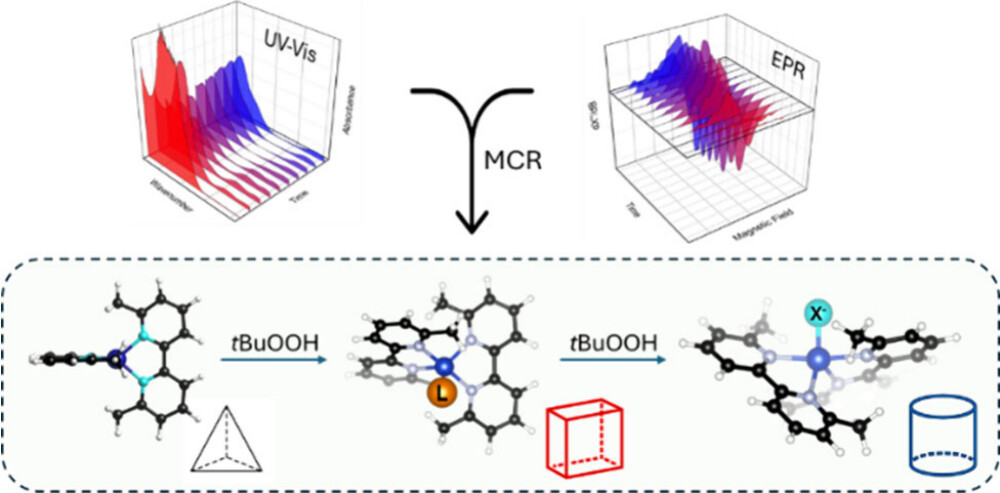
Despite their prevalence in catalysis, complexreaction mixtures are not trivial to investigate and disentangle.Different approaches can be applied to characterize them, evenfeaturing low-dimensionality data sets. The liquid-phase reaction of[CuI(6,6′-dimethyl-2,2′-bipyridyl)2]PF6 (CuI) with tert-butylhydroperoxide is investigated: two CuII species are found uponoxidation of the pristine complex, characterized by differentspectroscopic and kinetics fingerprints. Coupling EPR and UV−visspectroscopies with chemometric methods (namely, multivariatecurve reconstruction, MCR) allowed for easily retrieving purespectral features and concentration profiles. Spectrokinetic analysisindependently showed an optimal agreement with kinetic out-comes from MCR. Finally, hypotheses on the nature of the CuII species are drawn on the basis of EPR fitting and quantum chemistrycomputations on a series of candidate structures. Beyond the accurate characterization of a model system, this study demonstratesthe potential of coupling multivariate statistical techniques, experiments, and computations toward a quantitative understanding ofelectronic and kinetic information on complex chemical systems.
Click here for the complete article!